MODULE 10B - SLEEP HEALTH |
objectives
- Determine the anatomy and physiology of the circadian system.
- Examine the role of light exposure and endogenous melatonin in sleep health.
- Identify the effects of circadian dysregulation on physical and mental health.
- Explain how to identify, diagnose, and treat circadian disorders of the sleep-wake cycle.
chronobiology
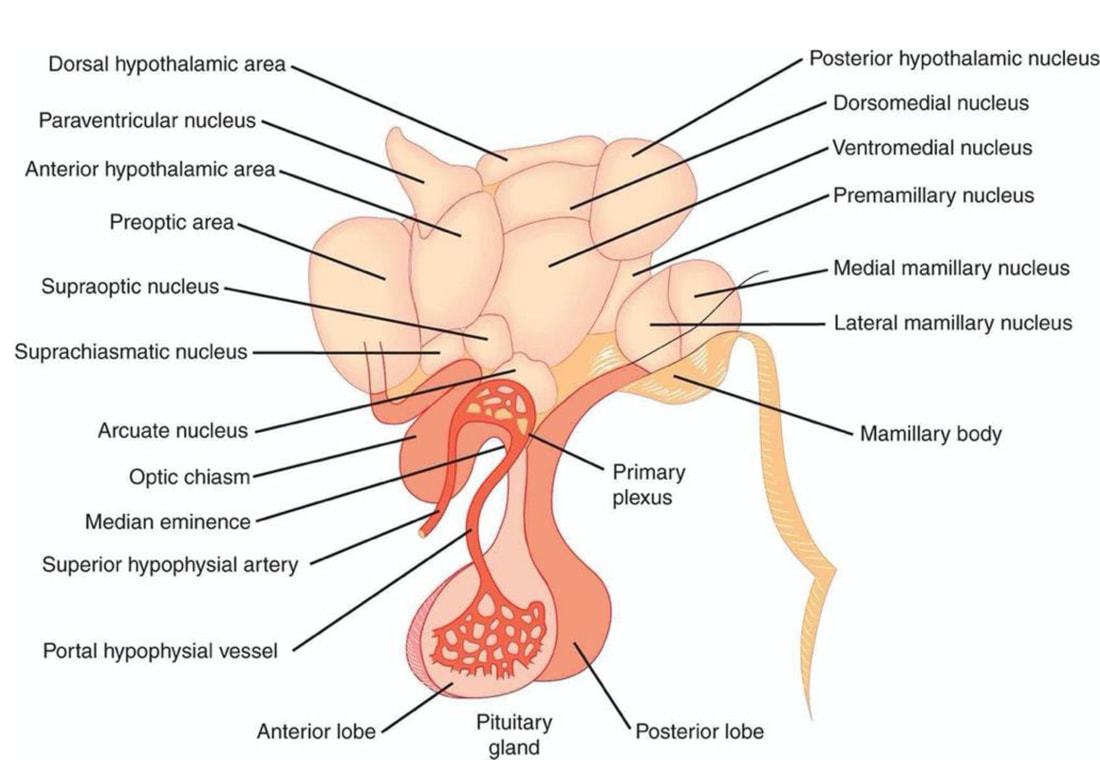
The master biologic clock regulating the timing of sleep and wake in mammals also regulates most if not all 24-hour (i.e., circadian) behavioral, physiologic, and biochemical rhythms. This master circadian clock is located in the bilaterally paired suprachiasmatic nucleus (SCN) in the anterior hypothalamus.
The expression of most rhythms at the behavioral, physiologic, and biochemical levels is regulated by the integration of inputs from the circadian clock and the sleep-wake state. Of particular note of the “downstream” rhythms regulated by the circadian clock is the feeding rhythm. In the last few years, a number of studies have demonstrated that the timing of food intake can regulate the expression of circadian clock genes in many peripheral tissues, which in turn control the 24-hour rhythm in the expression of many clock-controlled genes (perhaps as many as 10% to 50% of all genes expressed in a given tissue). The many interactions between the systems regulating circadian, sleep, and energy balance have led to much interest in the importance of these linkages to obesity, diabetes, and cardiometabolic disorders.
The longer one is awake or deprived of specific sleep stages, the greater will be the drive to recover the lost sleep or sleep stage. However, this may be true only after short periods of sleep deprivation because during continuous chronic partial sleep deprivations in rodents, there is a loss of the homeostatic recovery sleep increase, as the sleep-wake system appears to change from a homeostatic to an allostatic response system.
Our 24/7 society has led humans to be the only species that routinely ignores its biological clock as we are often awake when the clock is telling us to be asleep. Both the chronic disruption of circadian organization and chronic insufficient sleep have been associated with a wide range of mental and physical disorders. Modern medicine is only beginning to recognize that the treatment of many disorders of human health may need to take into account circadian science to improve overall 24-hour organization between and within the central nervous system and peripheral tissues.
The expression of most rhythms at the behavioral, physiologic, and biochemical levels is regulated by the integration of inputs from the circadian clock and the sleep-wake state. Of particular note of the “downstream” rhythms regulated by the circadian clock is the feeding rhythm. In the last few years, a number of studies have demonstrated that the timing of food intake can regulate the expression of circadian clock genes in many peripheral tissues, which in turn control the 24-hour rhythm in the expression of many clock-controlled genes (perhaps as many as 10% to 50% of all genes expressed in a given tissue). The many interactions between the systems regulating circadian, sleep, and energy balance have led to much interest in the importance of these linkages to obesity, diabetes, and cardiometabolic disorders.
The longer one is awake or deprived of specific sleep stages, the greater will be the drive to recover the lost sleep or sleep stage. However, this may be true only after short periods of sleep deprivation because during continuous chronic partial sleep deprivations in rodents, there is a loss of the homeostatic recovery sleep increase, as the sleep-wake system appears to change from a homeostatic to an allostatic response system.
Our 24/7 society has led humans to be the only species that routinely ignores its biological clock as we are often awake when the clock is telling us to be asleep. Both the chronic disruption of circadian organization and chronic insufficient sleep have been associated with a wide range of mental and physical disorders. Modern medicine is only beginning to recognize that the treatment of many disorders of human health may need to take into account circadian science to improve overall 24-hour organization between and within the central nervous system and peripheral tissues.
anatomy & physiology of the circadian system
- The master circadian clock for behavioral rhythms, including the sleep-wake cycle, is located in the suprachiasmatic nucleus (SCN).
- The SCN is situated in the anterior hypothalamus immediately dorsal to the optic chiasm and lateral to the third ventricle.
- Lesions of the SCN or its efferent projections abolish behavioral and endocrine rhythms, demonstrating a critical role for the SCN clock in generating the circadian rhythm of sleep.
- The SCN is divided into a core and a shell
- The SCN core receives most of the retinal input
- The output of SCN neurons is thought to be principally inhibitory, based on the observation that most clock cells contain GABA.
- The endogenous circadian rhythm of neuronal activity in the SCN is close to, but not exactly, 24 hours.
- To entrain to the imposed solar day length, the SCN clock must therefore be reset daily by extrinsic time cues, most notably the light-dark cycle.
- The SCN rhythm can be further tuned by nonphotic inputs to coordinate behavioral and physiologic patterns with diurnal changes in the environment.
- The SCN core receives dense input from the retina, intergeniculate leaflet, and midbrain raphe nuclei.
- The SCN shell primarily receives projections from other hypothalamic areas, basal forebrain, limbic cortex, septal area, and brainstem.
- The major neuronal pathway mediating the SCN-generated circadian rhythm of sleep is through a first-order projection to the subparaventricular zone (SPZ), followed by a second-order projection to the dorsomedial hypothalamic nucleus (DMH).
- The DMH projects heavily to brain areas involved in regulating sleep and wakefulness.
- The DMH sends primarily GABAergic projection to the ventrolateral preoptic nucleus (VLPO). The VLPO contains neurotransmitters GABA and galanin and is thought to promote sleep through its inhibitory projections to the ascending arousal systems.
- The DMH also sends primarily glutamatergic projection to the lateral hypothalamus, which contains wake-promoting neurons, including orexin-expressing neurons.
- In summary, the DMH receives circadian input from the SCN and the SPZ and projects to the VLPO and lateral hypothalamic area (LHA), defining a putative pathway for the circadian regulation of sleep and wakefulness.
- The relay of SCN circadian signals in the SPZ and then the DMH might allow for modification of circadian rhythms by other inputs such as food availability, external temperature, or social cues.
Circadian Regulation of Melatonin and Cortisol
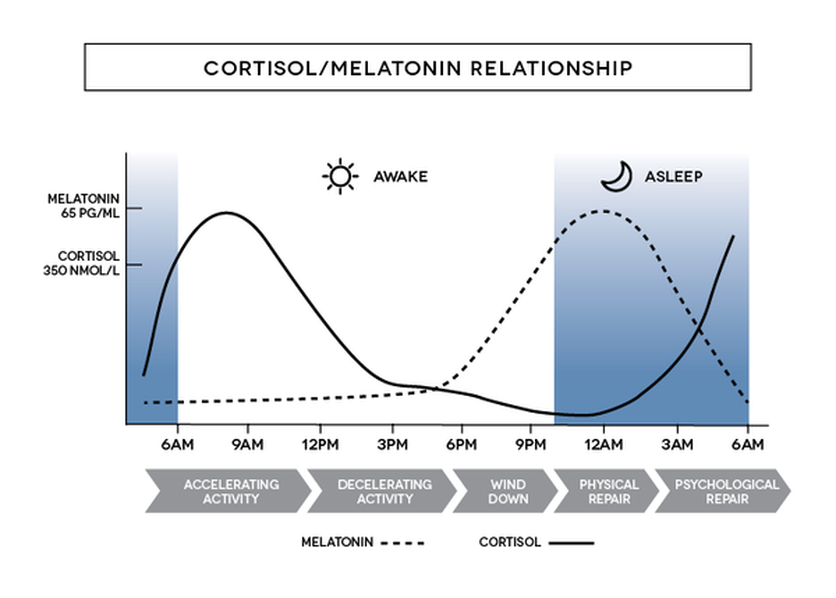
- Under normally entrained conditions, synthesis of melatonin usually begins a few hours before a person’s bedtime. Plasma melatonin levels are highest in the middle of the night and then decrease before usual wake time.
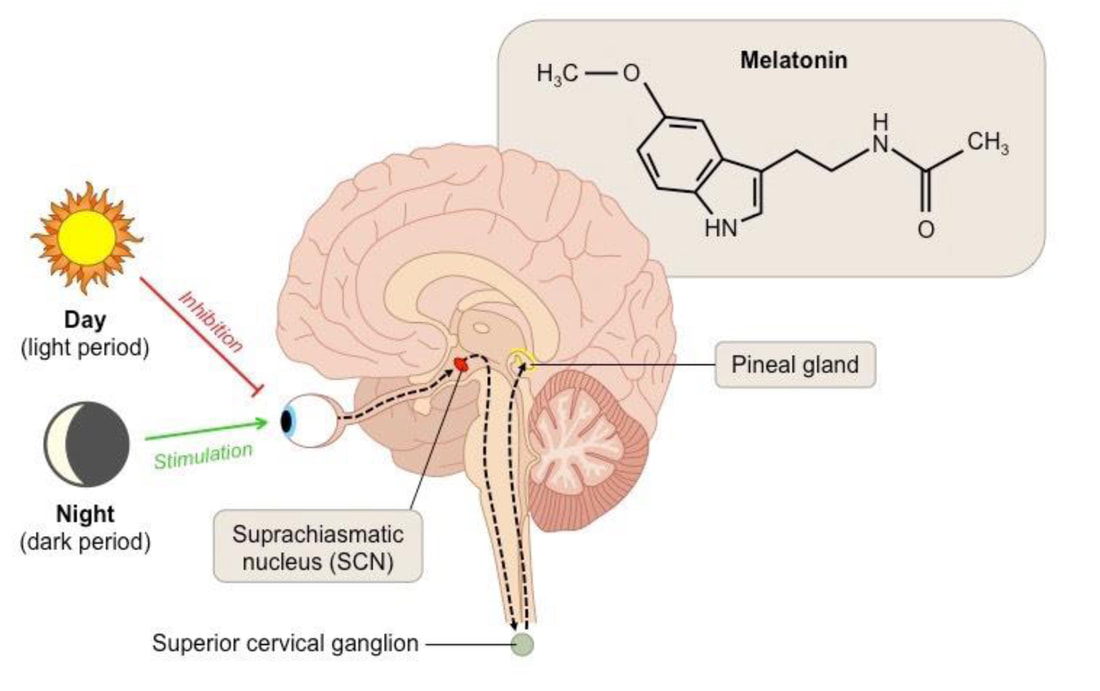
- The SCN is required for the circadian synthesis and release of melatonin in the pineal gland, which is regulated through a circuitous pathway.
- The SCN is most active during the biologic daytime and inhibits tonic activity of the paraventricular hypothalamic nucleus (PVH) neurons.
- Results in a lower firing rate in the pathway, effectively inhibiting melatonin synthesis.
- Therefore, melatonin is produced during the biologic night when SCN activity is low.
- Exposure to light during the biologic night activates SCN neurons through the retinohypothalamic tract (RHT), resulting in the inhibition of melatonin synthesis.
- Melatonin can also provide direct feedback to the circadian system via melatonin receptors (MT1 and MT2) located in the SCN. Melatonin acutely inhibits SCN electrical activity, and this response is mediated by the MT1 receptor.
- In humans, daily administration of melatonin can entrain the circadian system of blind persons, indicating that melatonin elicits phase shifts of the human circadian clock and can be used to treat circadian rhythm sleep disorders even in the absence of photoreception.
- The circadian rhythm of cortisol rises sharply before the waking period, presumably to promote a general state of readiness in anticipation of the myriad stressors and metabolic demands associated with the active phase.
- SCN neurons project directly and indirectly to the DMH, which sends efferents to the PVH and is critical for expression of the corticosteroid rhythm. Corticotropin-releasing hormone-containing neurons in the PVH project to the median eminence, where CRH is released into the portal circulation and activates the release of adrenocorticotropic hormone from the anterior pituitary gland. The SCN-driven release of ACTH in the blood results in rhythmic induction of corticosteroid secretion from the adrenal gland.
Synchronization of Central and Peripheral Oscillators
The suprachiasmatic nucleus is an internal body clock that allows daily scheduling of wake-sleep, feeding, corticosteroid secretion, and other bodily functions during a normal light-dark cycle. This cycle can be modified by light exposure or by administration of melatonin, which resets the body clock, but feeding schedules can also reset the daily activity cycle by acting on circuits that are downstream of the clock. Thus in patients with difficulty adjusting to shift work, jet lag, or other disruptions of their schedules, in addition to adjusting light exposure and taking melatonin, it is usually possible to adjust to a new schedule more quickly if the meal times, social interactions, and sleep-activity schedule of the new environment are adopted as soon as possible.
Synchronization of Central and Peripheral Oscillators
- The SCN contains a master clock for regulating behavioral rhythms, but circadian clocks are also found in tissues throughout the body.
- Damage to SCN neurons causes peripheral clocks to fall out of synchrony.
- Under most conditions, the SCN entrains peripheral clocks to ensure coordinated changes in physiology that are appropriately timed to the rest-activity cycle.
- There is evidence that shifts in temperature can reset peripheral, but not SCN, clocks. Thus by controlling the daily cycle of body temperature, the SCN would reset clocks throughout the body and keep them in synchrony.
- Entrainment of peripheral clocks by feeding cycles can potentially occur through nutrient-sensing pathways because proteins involved in metabolism and energy balance interact with core clock proteins and affect their function.
- Recent work suggests that the dietary composition and timing of meals can also affect the molecular clock and clock-controlled metabolic function.
The suprachiasmatic nucleus is an internal body clock that allows daily scheduling of wake-sleep, feeding, corticosteroid secretion, and other bodily functions during a normal light-dark cycle. This cycle can be modified by light exposure or by administration of melatonin, which resets the body clock, but feeding schedules can also reset the daily activity cycle by acting on circuits that are downstream of the clock. Thus in patients with difficulty adjusting to shift work, jet lag, or other disruptions of their schedules, in addition to adjusting light exposure and taking melatonin, it is usually possible to adjust to a new schedule more quickly if the meal times, social interactions, and sleep-activity schedule of the new environment are adopted as soon as possible.
summary
Exposure to light activates photopigment melanopsin (OPN4)-containing retinal ganglion cells (RGCs), which transmit light information to the core region of the SCN by releasing glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP). The effects of light on SCN neuronal activity are modulated by input from NPY- and GABA-containing neurons in the IGL and serotoninergic input from the MRN. The solar cycle entrains the master clock in the SCN, which is responsible for generating circadian patterns of behavior.
The SCN regulates the circadian sleep-wake rhythm through a primary projection to the SPZ, followed by a secondary projection to the DMH. The DMH, in turn, projects to brain areas critical for promoting sleep or wakefulness. A direct projection from the SCN to the PVH mediates rhythmic control of melatonin secretion from the pineal gland, and an indirect projection from the SCN to the PVH through the DMH is critical for the circadian release of corticosteroids. Although SCN neuronal projections are required for photic entrainment and circadian control of endocrine rhythms, SCN-diffusible factors are sufficient to support a weak circadian rest-activity rhythm.
The SCN also coordinates the timing of peripheral clocks, which respond to SCN-directed circadian changes in temperature, glucocorticoids, and nutrient-sensing pathways. Through the aforementioned pathways, the circadian system ensures that sleep and other biologic rhythms are timed appropriately with respect to daily changes in the environment.
CIRCADIAN SYSTEM AND SLEEP-WAKE REGULATION
Circadian clocks have several defining characteristics: a) endogenous rhythmicity that persists independent of periodic changes in the external environment, b) a near 24-hour period (circadian from Latin circa meaning “about” and dies meaning “day”), and c) the capacity for environmental input to modify or reset the timing or phase of the rhythm.
Influence of Sleep and Circadian Rhythms on Human Physiology
Effects of Light on Human Circadian Rhythms
Nonphotic Circadian Phase Resetting and Reentrainment
Nonphotic input to the circadian system is less well characterized than photic input.
Clinical Pearl
Disruption of the circadian timing system by shift work, travel across time zones, and the activities of our round-the-clock society has an adverse effect on many physiologic variables including hormones, glucose, metabolism, autonomic nervous system activity, the immune system, inflammation, neurobehavioral performance, and the propensity for, timing, and internal structure of sleep. This disruption can result in patient complaints of insomnia or excessive daytime sleepiness and can impair overall health.
The SCN regulates the circadian sleep-wake rhythm through a primary projection to the SPZ, followed by a secondary projection to the DMH. The DMH, in turn, projects to brain areas critical for promoting sleep or wakefulness. A direct projection from the SCN to the PVH mediates rhythmic control of melatonin secretion from the pineal gland, and an indirect projection from the SCN to the PVH through the DMH is critical for the circadian release of corticosteroids. Although SCN neuronal projections are required for photic entrainment and circadian control of endocrine rhythms, SCN-diffusible factors are sufficient to support a weak circadian rest-activity rhythm.
The SCN also coordinates the timing of peripheral clocks, which respond to SCN-directed circadian changes in temperature, glucocorticoids, and nutrient-sensing pathways. Through the aforementioned pathways, the circadian system ensures that sleep and other biologic rhythms are timed appropriately with respect to daily changes in the environment.
CIRCADIAN SYSTEM AND SLEEP-WAKE REGULATION
Circadian clocks have several defining characteristics: a) endogenous rhythmicity that persists independent of periodic changes in the external environment, b) a near 24-hour period (circadian from Latin circa meaning “about” and dies meaning “day”), and c) the capacity for environmental input to modify or reset the timing or phase of the rhythm.
- The suprachiasmatic nucleus in the anterior hypothalamus is the central neural pacemaker of the circadian timing system.
- Studies show that multiple distributed circadian oscillators drive daily rhythms in peripheral systems. These peripheral clocks use the same molecular machinery as the central circadian pacemaker in the SCN.
Influence of Sleep and Circadian Rhythms on Human Physiology
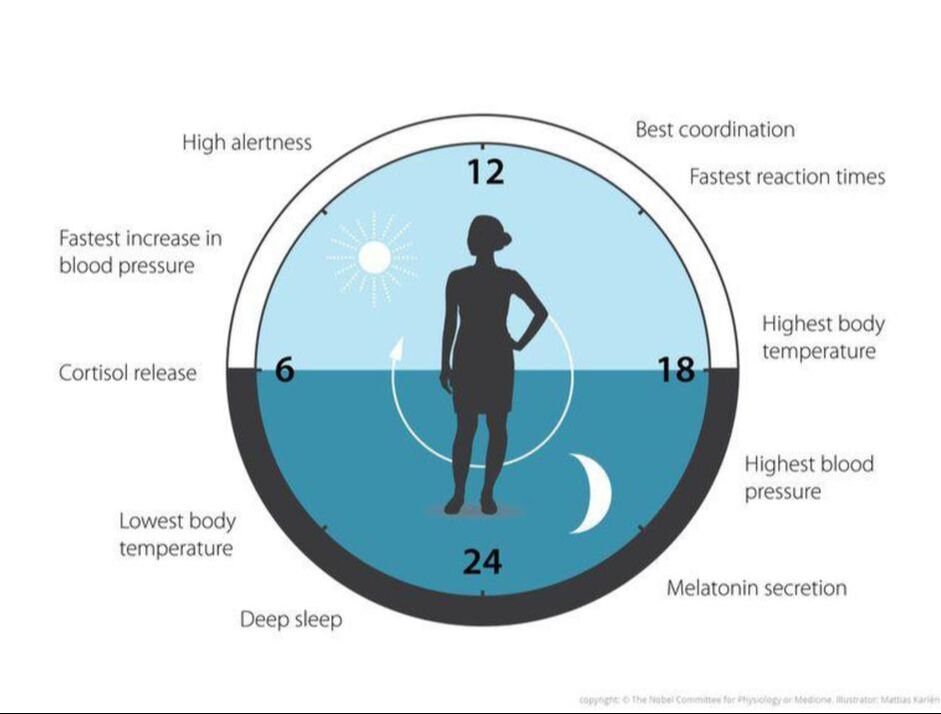
- Core body temperature is lowest and melatonin levels are highest during night sleep
- Cortisol is low at habitual sleep onset but high at habitual morning wake time.
- TSH levels are highest just before sleep onset and continue to be suppressed during the remainder of the sleep episode.
- Growth hormone, prolactin, and parathyroid hormone levels all show a prominent sleep-dependent increase.
Effects of Light on Human Circadian Rhythms
- The light-dark cycle is the primary environmental signal that synchronizes circadian systems in a wide array of species, including humans.
- Direct retinal input travels through the retinohypothalamic tract, a monosynaptic pathway by which information about the environmental light-dark cycle reaches the SCN.
- Only the ganglion cells that project from retina to SCN selectively contain the vitamin A-based photopigment melanopsin.
- Blue and short-wavelength green light (about 450 to 500 nm) is the most potent in shifting circadian phase in animals and for melatonin suppression and phase-shifting in humans.
- Both daytime and nighttime retinal exposure to such monochromatic blue (460 nm) light significantly improves reaction time, reduces attentional failures, and improves EEG correlates of alertness.
- In natural-light-only conditions, the internal circadian clock is synchronized to solar time with melatonin onset near sunset and melatonin offset before wake time and after sunrise, at a significantly earlier circadian phase.
- In contrast, evening reading from an electronic tablet that emits short-wavelength-enriched visible light delays endogenous circadian melatonin phase and the timing of REM sleep and increases evening alertness, sleep latency, and morning sleepiness compared with reading a printed book.
- Taken together, these findings suggest that artificial light between dusk and dawn alters physiology through the non-image-forming visual system by shifting circadian phase, inhibiting sleep-promoting neurons, activating arousal-promoting orexin neurons in the hypothalamus, and suppressing melatonin.
- These effects of nocturnal artificial light in turn mask sleepiness, transiently increase alertness, and directly interfere with sleep, leading to chronic sleep deficiency.
Nonphotic Circadian Phase Resetting and Reentrainment
Nonphotic input to the circadian system is less well characterized than photic input.
- Exposure of healthy young men to nocturnal exercise of 1 to 3 hours duration resulted in phase delays in nocturnal melatonin the following day.
- Late afternoon exercise has phase-advancing effects on the circadian clock. Participants exhibited partial entrainment, advancing an average of 10 minutes per day more than non-exercising controls.
Clinical Pearl
Disruption of the circadian timing system by shift work, travel across time zones, and the activities of our round-the-clock society has an adverse effect on many physiologic variables including hormones, glucose, metabolism, autonomic nervous system activity, the immune system, inflammation, neurobehavioral performance, and the propensity for, timing, and internal structure of sleep. This disruption can result in patient complaints of insomnia or excessive daytime sleepiness and can impair overall health.
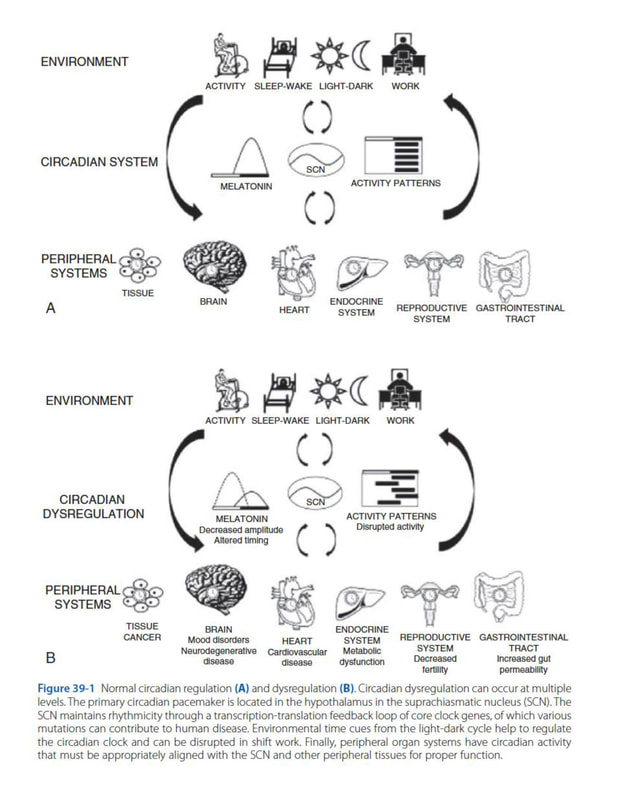
circadian regulation in mental and physical health
Circadian rhythms are essential for the coordination of physiologic and behavioral functions with the 24-hour environment. Therefore disruption to the circadian system can result in a broad range of adverse mental and physical health consequences.
Melatonin is a key marker of circadian function. Melatonin is secreted by the pineal gland, and levels peak in the middle of the night. Light suppresses the normally observed nocturnal rise in melatonin. Melatonin can be measured in the blood, saliva, and urine, and both the timing and amplitude of secretion can be evaluated as measures of circadian alignment.
Shift Work and Social Jet Lag shift work is a major source of circadian dysregulation because workers are required to be awake during times when they would normally sleep. Many live in a chronic state of varying degrees of circadian misalignment, being awake at night on workdays and during the daytime on days off because of social and family responsibilities. A large number of individuals are required to wake up much earlier on workdays than they would prefer to, then sleeping in on non-workdays. The mismatch between work and nonwork sleep times is referred to as social jet lag. There is growing evidence that the circadian misalignment associated with shift work or social jet lag contributes to the development of disease , with the strongest evidence for an increased risk for cancer and cardiometabolic disorders.
Melatonin is a key marker of circadian function. Melatonin is secreted by the pineal gland, and levels peak in the middle of the night. Light suppresses the normally observed nocturnal rise in melatonin. Melatonin can be measured in the blood, saliva, and urine, and both the timing and amplitude of secretion can be evaluated as measures of circadian alignment.
Shift Work and Social Jet Lag shift work is a major source of circadian dysregulation because workers are required to be awake during times when they would normally sleep. Many live in a chronic state of varying degrees of circadian misalignment, being awake at night on workdays and during the daytime on days off because of social and family responsibilities. A large number of individuals are required to wake up much earlier on workdays than they would prefer to, then sleeping in on non-workdays. The mismatch between work and nonwork sleep times is referred to as social jet lag. There is growing evidence that the circadian misalignment associated with shift work or social jet lag contributes to the development of disease , with the strongest evidence for an increased risk for cancer and cardiometabolic disorders.
- The Nurses’ Health Study found that a cohort of women who worked on the night shift for 30 or more years had a relative risk of 1.36 for developing breast cancer, whereas a cohort of 4036 Swedish women found a hazard ratio of 2.02 for developing breast cancer if working shifts that included night work.
- A meta-analysis of 28 studies evaluating circadian disruption and breast cancer risk found that the relative risk for breast cancer was 1.19 for shift work.
- Shift work has also been associated with an increased risk for prostate cancer, ovarian cancer, lung cancer, and non-Hodgkin lymphoma.
- Overall these results prompted the International Agency for the Research on Cancer in 2007 to conclude that shift work involving circadian disruption is probably carcinogenic (group 2A).
- However, several studies have not found an association between shift work and breast cancer, including a cohort of 73,029 Chinese women surveyed regarding lifetime shift work exposure and breast cancer.
- A study that included 27,485 workers in Sweden found an increase in obesity, elevated triglycerides, decreased HDL, and impaired glucose tolerance among shift workers.
- It has also been found that shift work is associated with an increase in systolic and diastolic blood pressure and with a loss of the normal nocturnal dip in blood pressure.
- Prospective trials have found that both men and women working rotating shifts have an accelerated development of metabolic syndrome compared with day workers.
circadian dysregulation & psychiatric disorder
Many psychiatric disorders have been noted to have a component of circadian dysregulation associated with them, with the strongest evidence for mood disorders. There is growing evidence that treatments based on addressing circadian dysregulation can be effective adjunctive treatment for treating several psychiatric disorders.
Seasonal Affective Disorder
Depression
Bipolar Disorder
Seasonal Affective Disorder
- Consists of depressive symptoms that are primarily present in the late fall and winter, during a period of shorter days when there is decreased exposure to natural bright light as well as decrease in the intensity of light that is available.
- Studies suggest that it is not only a lack of exposure to light but also a decreased ability to respond to light that contributes to the underlying pathology.
- The best studied and most widely accepted of circadian-regulation treatment for psychiatric disorders is bright light therapy for the treatment of SAD.
- SAD symptoms are symptoms are greater in individuals with a larger degree of circadian misalignment, and response to treatment corresponds with the degree of realignment.
Depression
- Often associated with early morning awakenings and decreased REM sleep latency
- Light therapy is generally not considered first-line treatment, but it can be successfully used as an adjunctive therapy for patients with drug-resistant depression or for individuals for whom medications may be undesirable.
Bipolar Disorder
- Exhibit recurring cycles of depression and mania or hypomania
- Evidence of greater evening preference and decreased stability of the sleep-wake pattern
- Interpersonal and social rhythm therapy is a successful treatment strategy that, along with standard medication therapy, focuses on behavioral strategies to address patients’ sleep and circadian vulnerabilities.
circadian disorders of sleep-wake cycle
Delayed Sleep-Wake Phase Disorder
Pathogenesis
Diagnosis
Treatment
Advanced Sleep-Wake Phase Disorder
Pathogenesis
Diagnosis
Treatment
- DSWPD is characterized by sleep onset and wake times that are usually delayed more than 2 hours and often up to 3-6 hours relative to conventional sleep-wake times.
- The typical patient finds it difficult to initiate sleep before 2-6 AM and, when free of societal constraints, prefers wake times of 10 AM to 1 PM.
- Sleep itself is normal for age
- Symptoms are chronic, usually at least 3 months – and quite often of many years duration.
- Patients are unable to advance their sleep times despite repeated attempts
- More common in adolescents and young adults with a prevalence of 3.3% to 7%.
- In a sleep disorders clinic, 6.7% to 16% of patients seen for a primary complaint of insomnia were determined to have DSWPD.
Pathogenesis
- Behavioral preference may play a major role in some cases of DSWPD
- In adolescence, for example, the biologic delay in the timing of circadian rhythms is likely exacerbated by late evening activities, such as doing homework, watching TV, and using the Internet.
- Other factors: use of caffeine, artificial light at night, late wake times resulting to delayed light exposure in the morning.
- There is evidence that some individuals with DSWPD have a hypersensitivity to nighttime suppression of melatonin by bright light.
- Impaired sleep recovery
Diagnosis
- Patient’s history of chronic or recurrent complaint of symptoms of insomnia due to a stable delay in the timing of the major sleep and wake period.
- The sleep disturbance is associated with impairment of social, occupational, or other areas of functioning.
- Sleep log or actigraphy monitoring should be performed for at least 7 days, but preferably 14 days to demonstrate a stable delay in the timing of the habitual sleep period.
- Medical, mental, or sleep disorders that may cause alterations in the sleep-wake cycle, insomnia, or excessive sleepiness should be excluded or adequately treated.
- Polysomnography is not necessary to establish diagnosis but should be performed when another primary sleep disorder is suspected.
Treatment
- Chronotherapy: requires a successive delay of sleep times by 3 hours daily over a 5- to 6-day
- Light Therapy: therapy with bright light (10,000 lux for 30-45 minutes) in the morning (1-2 hours shortly after awakening) should advance the phase of circadian rhythms in DSWPD and may be more practical than chronotherapy.
- Melatonin: 0.3 to 5 mg 5-8 hours before bedtime
- In recent studies, combination of bright light after awakening and melatonin 8 hours before bedtime resulted in larger advances than either melatonin or light alone.
- Sleep hygiene
- Avoidance of exposure to bright light in the evening
Advanced Sleep-Wake Phase Disorder
- ASWPD is characterized by habitual and involuntary sleep and wake times that are usually more than 2 hours earlier than societal averages.
- Sleep itself is normal for age.
- Individuals frequently complain of persistent and often irresistible sleepiness in the late afternoon or early evening, often preventing their participation in desired evening activities.
- Involuntary early morning awakening (2-5 AM), which occurs even if sleep onset is voluntarily delayed.
- Because of professional or social obligations, later bedtimes can lead to chronically insufficient sleep and excessive daytime sleepiness.
- In general, people with ASWPD have less difficulty adjusting to the earlier schedule than those with DSWPD because societal constraints on sleep time are less rigid than on wake time.
Pathogenesis
- Etiology is not well understood
- Possibly an abnormal phase response curve to light or due to a shortened endogenous period (less than 24 hours).
- Several familial cases of ASWPD have been reported in the literature. These families show a clear autosomal dominant mode of inheritance.
Diagnosis
- Made primarily on the basis of clinical history
- Associated with impairment of social, occupational, or other areas of functioning
- Sleep log or actigraphy monitoring for at least 7 days and preferably 14 days should be performed
- Other medical, mental, or sleep disorders that may cause alterations in the sleep-wake cycle, insomnia, or excessive sleepiness should be excluded or adequately treated.
- Polysomnography is not necessary to establish diagnosis but should be performed when another primary sleep disorder is suspected.
Treatment
- Chronotherapy: advance bedtime by 3 hours every 2 days until the desired bedtime is reached
- Light Therapy: bright light therapy during early evening (7-9 PM)
- Melatonin: given in the early morning, usually on awakening
- However, evidence for its effectiveness or safety in the treatment of patients with ASWPD is generally lacking. It should be noted that the sedating effects of melatonin, which can be variable in patients, may limit its usefulness in this regard.
REVIEW QUESTIONS
1. What is the location of the master circadian clock?
a. Anterior hypothalamus
b. Posterior hypothalamus
c. Ventrolateral Preoptic Nucleus
d. Optic chiasm
2. Which of the following is true regarding the SCN?
a. Activity is stimulated by melatonin
b. The SCN core receives most of the retinal input
c. It is most active during nighttime
d. Reset by the light-dark cycle and changes in temperature
3. According to studies, which condition/s are associated with shift work?
a. Breast cancer
b. Prostate cancer
c. Impaired glucose intolerance
d. All of the above
4. How is DSWPD diagnosed and managed?
5. How is ASWPD diagnosed and managed?
Answers
1. What is the location of the master circadian clock?
a. Anterior hypothalamus
b. Posterior hypothalamus
c. Ventrolateral Preoptic Nucleus
d. Optic chiasm
2. Which of the following is true regarding the SCN?
a. Activity is stimulated by melatonin
b. The SCN core receives most of the retinal input
c. It is most active during nighttime
d. Reset by the light-dark cycle and changes in temperature
3. According to studies, which condition/s are associated with shift work?
a. Breast cancer
b. Prostate cancer
c. Impaired glucose intolerance
d. All of the above
4. How is DSWPD diagnosed and managed?
5. How is ASWPD diagnosed and managed?
Answers
- A.
- B.
- D.
- Refer to module
- Refer to module
ARTICLE REVIEW
TASK - Reflective Journal |
Topics covered:
|
References:
- Fuhr L, Abreu M, Pett P, Relogio A. Circadian systems biology: when time matters. Comput Struct Biotechnol J 2015;13:417-26.
- Kryger, Meir, et al, Principles and Practice of Sleep Medicine, 6th ed. Philadelphia, USA: Elsevier, Inc., 2017.